- Home
- Contact Us
- News & Events
- Awards
- AAEES Awards Criteria
- 40 Under 40 Recognition Program
- Edward J.Cleary Award
- Excellence in Environmental Engineering and Science Education
- Gordon Maskew Fair Award
- Honorary Member
- International Honorary Member
- Ralph and Joe Bales Graber Science Award
- Stanley E. Kappe Award
- Environmental Communications Awards Competition
- Excellence in Environmental Engineering and Science Competition
- The AAEES Chapter Blue Marble Award
- Resources
- AAEES Microcredentials
- Annual Reports
- AAEES Press Releases
- AAEES Website How To VIdeos
- Environmental Engineer and Scientist
- Environmental Engineering Body of Knowledge
- PFAS Resources
- Specialty Examination Guide
- Students and Young Professionals Resources
- Who's Who in Environmental Engineering & Science®
- Leadership Opportunities
- Membership
- Donate
- Jobs
2025 Excellence in Environmental Engineering and Science® Awards Competition Winner
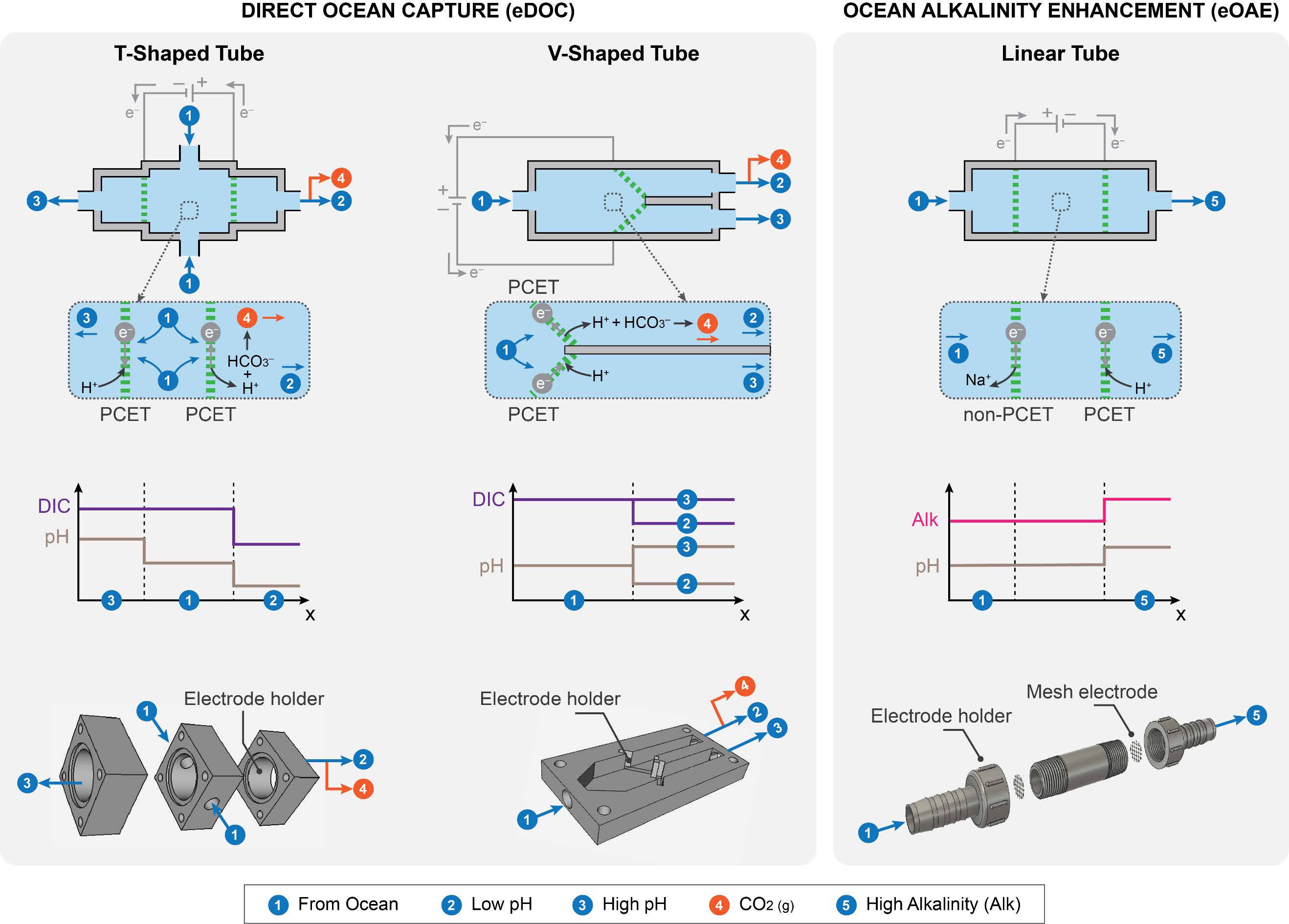
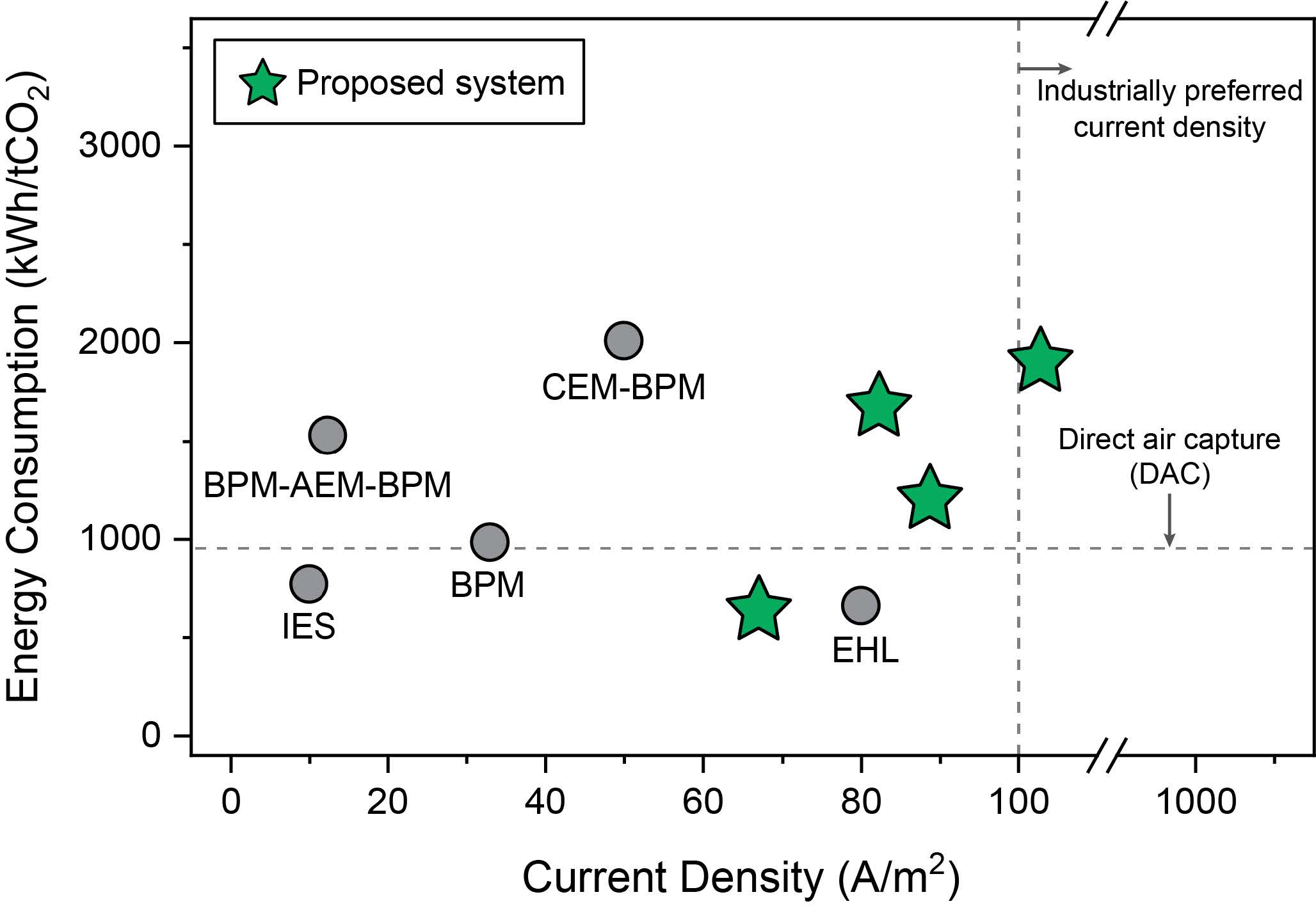
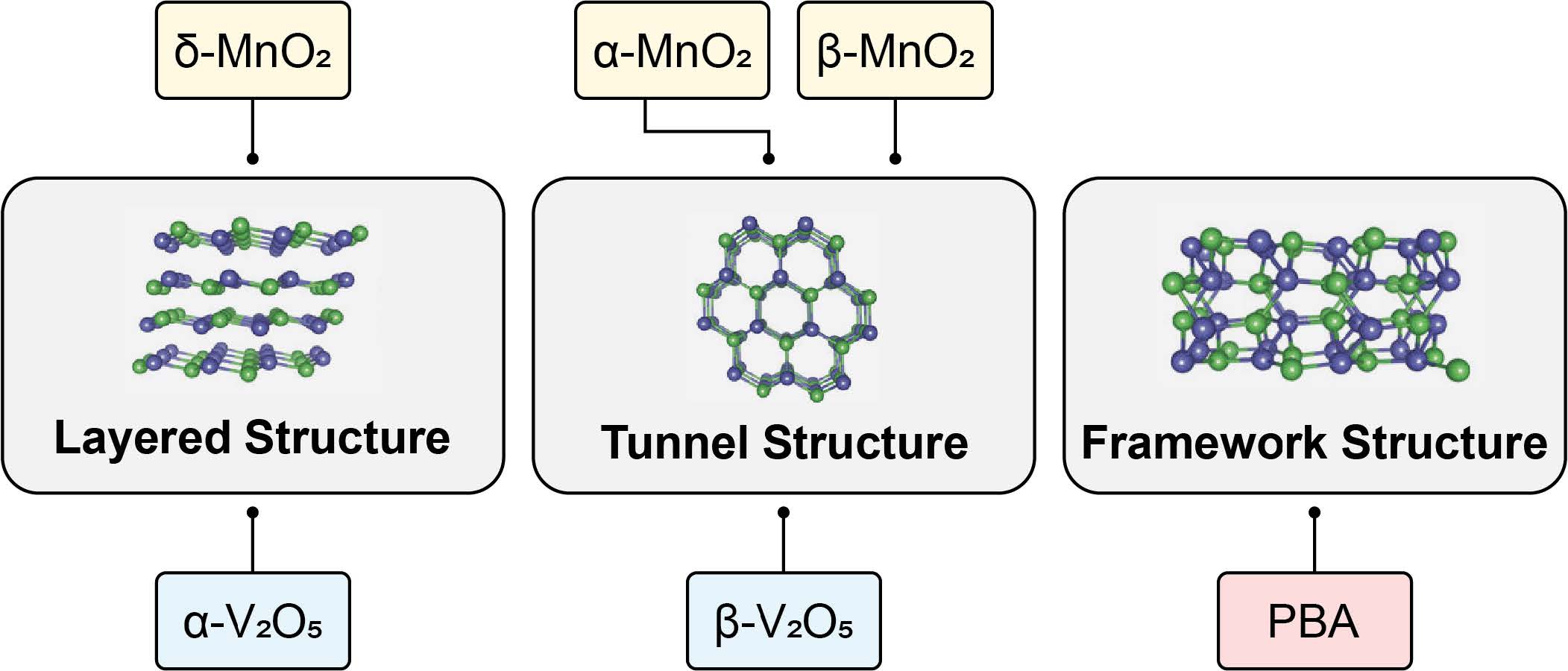
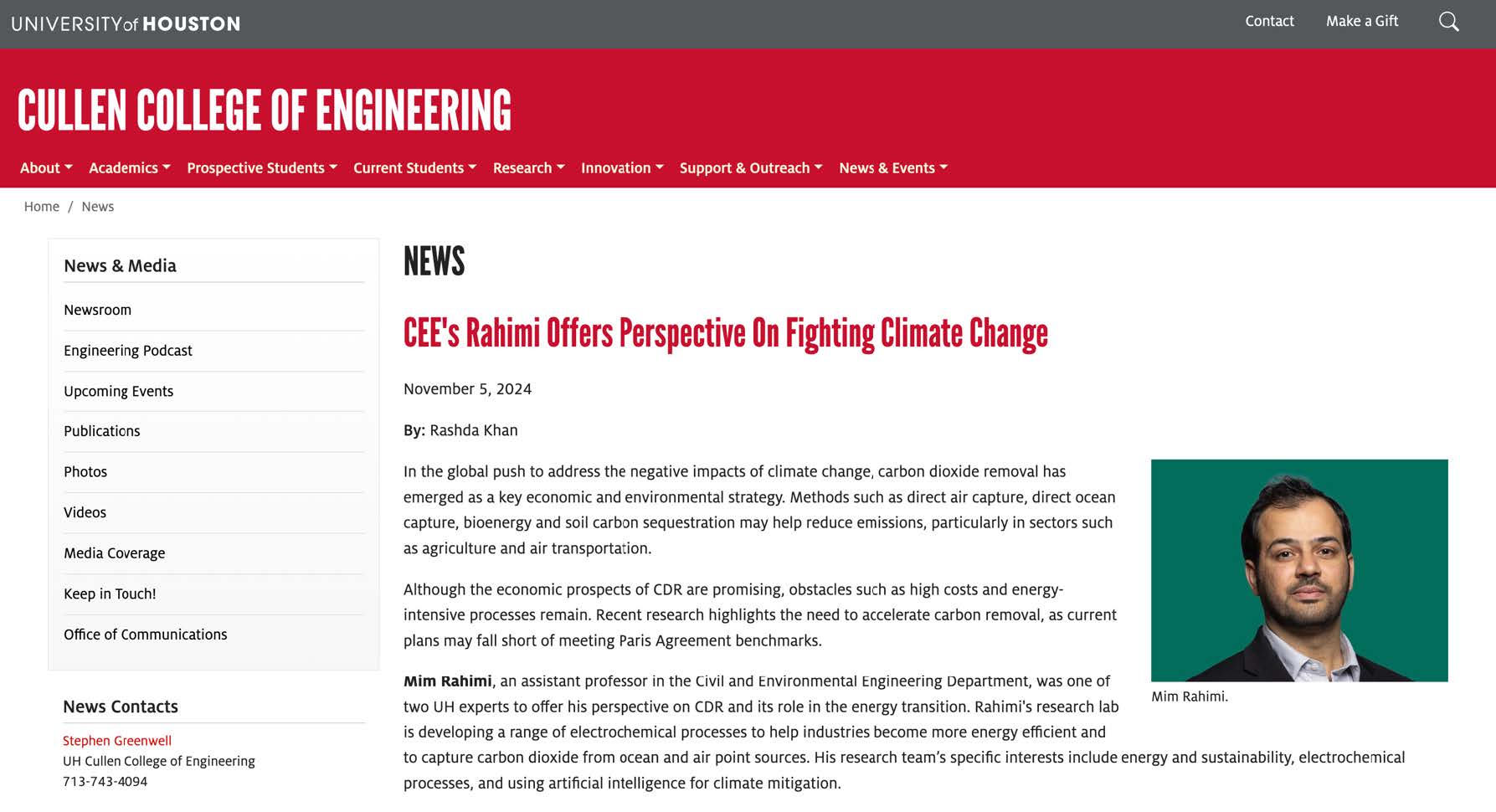
Honor Award - University ResearchElectrochemical Frontiers in Marine Carbon Dioxide RemovalEntrant: University of Houston Entrant ProfileDr. Mim Rahimi is an Assistant Professor of Environmental Engineering at the Cullen College of Engineering at the University of Houston (UH), where he also holds an affiliate appointment with the Materials Science and Engineering Program. Before joining UH, Dr. Rahimi was a postdoctoral associate in the Department of Chemical Engineering at the Massachusetts Institute of Technology (MIT) (2018– 2021), where he worked with Prof. Alan Hatton on advanced electrochemical systems. Dr. Rahimi earned his Ph.D. in Chemical Engineering from Pennsylvania State University in 2017 under the mentorship of Prof. Bruce Logan. At UH, Dr. Rahimi leads a research group dedicated to developing innovative electrochemical technologies for carbon capture from air, ocean, and industrial point sources. His team focuses on scalable and sustainable solutions to address global challenges in environmental engineering. Dr. Rahimi’s contributions have been recognized through several prestigious awards, including the 2024 NSF CAREER Award, the Department of Energy (DOE) National EnergyTech University Prize, and the UH-Chevron Energy Transition Innovation Challenge. In addition, he was recently honored as a member of the AAEES 40 Under 40 Recognition Program for his impact on environmental science and engineering. Beyond research, Dr. Rahimi is actively engaged in education and outreach. He has developed courses on energy and environmental systems, co-organized climate change workshops, and created innovative educational tools like the GigaCapture board game. For more information about Dr. Rahimi’s research and activities, please visit www.TeamRahimi.com. Project DescriptionINTRODUCTION AND MOTIVATIONThe increase in extreme climate events worldwide is closely associated with the rise in atmospheric carbon dioxide (CO2), largely due to human activities. There is a global consensus on the necessity of eliminating net carbon emissions to mitigate significant negative impacts. While reducing and capturing CO2 at its source remains crucial, the Intergovernmental Panel on Climate Change has emphasized the indispensability of environmental carbon dioxide removal (CDR) for atmospheric CO2 reduction [2]. However, the challenge lies in the lack of large-scale, effective, economical, and secure CDR methods, as well as the foundational science for their development. Recognizing these challenges and critical needs for innovative processes, this proposal introduces a marine-based CDR (mCDR) approach. The focus is on removing CO2 from the ocean, through either extraction of dissolved inorganic carbon or enhancement of ocean alkalinity. Emerging Electrochemical Processes for Carbon Capture. Electrochemically mediated methods have emerged in recent years as a promising unconventional approach for carbon capture [3]. Electrochemicalcarbon capture (ECC), primarily designed for capture from point sources like power plants, are increasingly being adapted for CO2 separation from dilute sources such as air and ocean. PI Rahimi has made significant contributions in this evolving field, particularly in developing ECC for a range of sources. Rahimi's work has resulted in notable publications and has been recognized through DOE grants and the NSF CAREER award. The development of ECC processes has catalyzed a resurgence in recognizing the ocean as a significantnatural carbon sink. Initial efforts in developing electrochemical processes for mCDR have focused on manipulating seawater pH. This process either concentrates dissolved inorganic carbon (DIC) into CO2 gas at low pH or precipitates it as magnesium or calcium carbonates at high pH. Most developed electrochemical processes rely on water electrolysis through a bipolar membrane configuration tomanipulate the seawater pH, generating acid and base streams. However, our recent in-depth analysis of these systems has identified significant challenges for large-scale mCDR implementation [1]: (1) The developed methods require high energy demand and a substantial need for rare metal catalysts. Our findings suggest that to construct a mCDR facility capable of processing 1 MtCO2 annually, about 20-30% of the world's platinum production would be required. Additionally, due to low current densities, thesesystems need a vast electrode area, roughly five times the size of Central Park in New York City, for the same removal capacity. (2) The developed approaches rely on multiple bipolar and ion-selective membranes. These membranes are not only expensive but also particularly prone to fouling, especially in seawater conditions, which contain a complex mix of biological and chemical species. Fouling issues have been evident even in lab-scale setups. This problem can significantly affect the performance and durability of membranes in real-world mCDR applications. The Proposed Electrochemical Process for mCDR. In this proposal, we introduce a fully membraneless electrochemical system with an innovative electrode design for mCDR applications. Similar to the previous systems, the redox reactions result in adjusting the seawater pH. However, our proposal significantly redesigns the nature of these reactions, the electrodes, and the cell configuration and operation. Instead of relying on water electrolysis, our system is based on proton-coupled electron transfer (PCET) reactions on intercalating electrodes. These electrodes act as proton hosts during the redox reaction, a novel approach compared to traditional methods. Limited research, including work by PI Rahimi, has explored intercalating electrodes for electrochemical carbon capture [4, 5]. However, these investigations, confined to a handful of materials (MnO2, Bi, Ag), were mainly proof-of-concept studies. These previous investigations did not systematically explore electrode design andmechanisms, nor did they extensively test a range of host materials. We also propose innovative flow-through electrochemical cell configurations, entirely eliminating the need for membranes. This configuration, showing promise in other applications like hydrogen electrolyzers, offers uniqueopportunities for developing membraneless systems suited to mCDR. The proposed membraneless cellswith intercalating electrodes represent an innovative solution to the limitations of current electrochemical mCDR systems, addressing issues such as the need for rare metal electrodes and the high costs and stability concerns associated with membranes. The proposed membraneless systems for mCDR are designed for two scenarios: electrochemical direct ocean capture (eDOC) and electrochemical ocean alkalinity enhancement (eOAE) (Figure 1). In the eDOC process, the anode compartment of a flow-through cell with symmetrical electrodes (using V- or T-shaped configurations) enables DIC removal through pH reduction and subsequent CO2 gas formation as a result of a PCET reaction. High pH generated by the opposite PCET reaction on the cathode most likely results in DIC removal through calcium and/or magnesium carbonate precipitations. Preliminary results using a V-shaped cell equipped with initially unoptimized MnO2 electrodes have demonstrated promising outcomes in terms of energy requirements for CO2 removal and current density, a direct indicator of the kinetics of the process, when compared to other electrochemical approaches developed for mCDR (Figure 2). In the eOAE process, asymmetrical intercalating electrodes are used in a tubular flow-through cell, which is conceptually similar to plug flow reactors in chemical systems. The primary aim is to produce seawater streams with enhanced alkalinity at the tube outlets, achieved by utilizing a non-PCET reaction on the cathode (e.g., using sodium-intercalating materials) and a PCET reaction on the protonintercalating anode. The enhanced alkalinity stream is then reintroduced into the ocean for further removal of CO2 from the atmosphere. Therefore, this method can be viewed as indirect air capture. To prevent precipitation, inlet streams will be pre-filtered to remove calcium and magnesium ions. In all configurations, electrode polarity will be periodically switched to prevent the depletion of intercalating species. These innovative configurations have the potential to significantly advance the field of mCDR. OBJECTIVESObjective 1: Advancing Electrode Material Design for Enhanced mCDR PerformanceHypothesis: By employing rational design principles, we can substantially improve energy efficiency andreduce the costs of the proposed mCDR through the development of optimized electrode materials. Objective 2: Mastering Interfacial Processes Across Multiple Length ScalesHypothesis: By conducting an in-depth investigation of the electrode-electrolyte interfaces at various length scales, we can gain a comprehensive understanding of the reaction mechanisms and charge transport processes when electrode materials interact with seawater. This knowledge will enable the design of high-performing electrodes for mCDR processes. Objective 3. Optimizing mCDR Process Dynamics and Reactivity RegenerationHypothesis: By optimizing operational parameters, and cell geometry, and implementing strategies for continuous regeneration of surface reactivity, we can maximize the carbon removal rate, minimize energy consumption, and mitigate the diminishing driving forces that impair capture kinetics and electrode surface activity in mCDR processes. TECHNICAL APPROACHA broad range of inorganics will serve as electrode materials, chosen for their exceptional electrochemical performance and structural flexibility, enabling high selectivity for our specific application. The primary charge storage mechanism inthese materials is an electrochemical intercalation reaction. Research has shown that the arrangement of ion channels within the material significantly influences the rate and selectivity of cation intercalation [6]. Consequently, we will explore three distinct crystal structures: layered, tunnel, and framework types. Our systematicinvestigation will include an extensive range of materials (Figure 3), focusing on crystal form engineeringto enhance performance. A comprehensive set of electrochemical measurements will be carried out to meet the objectives outlined, ranging from macro to microlevels. At the macroscale, conventional electrochemical techniques such as cyclic voltammetry and electrochemical impedance spectroscopy will be utilized. The primary aim is to evaluate the macroscopic properties of various electrode materials exposed to seawater. This includes understanding redox reactions and polarization profiles and conducting impedance analyses. For microscale investigations, high- resolution analysis will be employed to examine reaction mechanisms, interface phenomena, intercalation efficiency, and identify any parasitic reactions. These analyses will beconducted using scanning electrochemical microscopy (SECM), available in PI Rahimi's lab. SECM offers high spatial resolution and current sensitivity (down to a few femtoamperes) and facilitates single-entity electrochemistry. Concurrently, a range of integrated in-situ experiments will be performed tofurther evaluate the components and efficacy of the process. Our in-situ experimental framework includes techniques such as spectroelectrochemistry (SEC), electrochemical quartz crystal microbalance (eQCM), and electrochemical-mass spectrometry (EC-MS). Together, these in-situ analyses provide thecomprehensive data required to delve into both the fundamental and practical aspects of the mCDR process, ensuring a thorough understanding and optimization of the system. ALIGNMENT WITH JUDGING CRITERIAComprehensive, Integrated Approach. The proposed membraneless electrochemical systems for mCDR represent an integrated solution that directly addresses challenges in air, water, and oceanic systems. By leveraging the ocean's role as a natural carbon sink, the project mitigates atmospheric CO2 and enhances ocean alkalinity, thus contributing to the health of marine ecosystems. This dual approach integrates environmental media in a holistic manner, ensuring a broad environmental impact. Quality and Proven Performance. Preliminary results using unoptimized MnO2 electrodes in a V-shaped cell configuration have already demonstrated reduced energy requirements and improved currentdensities compared to existing technologies. These findings provide evidence of the system's performance potential. The proposal also builds upon established electrochemical techniques, ensuring rigorous scientific discipline and a high likelihood of achieving the outlined objectives. Originality and Innovation. This project introduces innovative concepts in the field of mCDR, including the development of PCET reactions on intercalating electrodes and membraneless flow-throughcell designs. These advancements address critical limitations of current mCDR technologies, such as highenergy demands, reliance on rare metals, and membrane fouling. Complexity of the Problem. The project tackles the multifaceted challenges of large-scale mCDR implementation, including energy efficiency, material design, and operational stability. By addressing issues such as the scarcity of rare materials, electrode performance under seawater conditions, and theoptimization of interfacial processes, the research demonstrates a high level of technical and scientific complexity. Contribution to Environmental, Social, and Economic Advancement. The proposed system offers scalable, energy-efficient solutions for reducing atmospheric CO2, with significant implications for mitigating climate change. Furthermore, the project contributes to economic sustainability by reducingreliance on costly materials and enhancing process efficiency. Socially, the research supports the broader goal of achieving global carbon neutrality, benefiting communities worldwide by addressing the pressing issue of climate change. REFERENCES 1. Lee, et al., Synthesis report of the IPCC Sixth Assessment Report (AR6), Longer report. IPCC. 2023; 2. Rahimi et al., Chemical Society Reviews, 2022. 51: 8676-8695; 3. Aleta ... Rahimi, Energy & Environmental Science, 2023. 16: 4944-4967; 4. Rahimi et al., Cell Reports Physical Science, 2020. 1(4): 100033; 5. Kim etal., Energy & Environmental Science, 2023. 16(5): 2030-2044; 6. Wu et al., Nature Reviews Materials, 2023. 8(1): 41-53. Click images to enlarge in separate window.
Click here to return to the list of 2025 winners. |